Abdominal imaging
Our imaging research in abdominal imaging focuses on the following main topics: chronic kidney disease (CKD) including diabetic kidney disease (DKD) and autosomal dominant polycystic kidney disease (ADPKD), chronic liver disease and drug-induced liver injury (DILI).
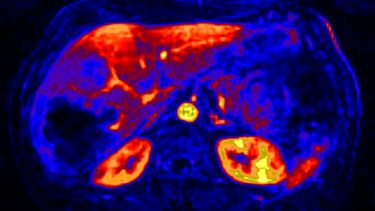
Our research in these areas covers; development of dedicated imaging biomarkers for these applications, improved assays and clinical studies to evolve their potential utility.
Chronic Kidney Disease (CKD)
The rising prevalence of CKD poses a major public health challenge affecting >10% of the population. However, the field has seen few new treatments, and an alarming number of large recent CKD progression trials have failed. In order to overcome these challenges, there is an urgent need for better biomarkers to identify patients that are at risk of progression, or are likely to respond to candidate therapeutics. We believe MRI biomarkers have a high potential to help fill this gap as they are non-invasive and sensitive to CKD pathophysiology.
Our research in this space covers a number of areas, including the development of novel methods such as a technique to measure single-kidney function, developed with funding from Kidney Research UK, and applicable in assessment of renal artery stenosis and healthy donors. We are also a main contributor to the setup of a national infrastructure to integrate advanced MRI in clinical trials (UKRIN-MAPS), where our role is to set up a central image processing facility. We are currently also taking this role forward in the NIHR study AFiRM that will recruit 500 CKD patients and follow them up with MRI. We are also leading an EU-wide network that aims to promote international coordination of research in this space.
Autosomal dominant polycystic kidney disease (ADPKD)
Our ADPKD research aims to improve the understanding of progression in patients with ADPKD, particularly at an early stage, to optimise opportunities for intervention to facilitate timely access to treatment or enrolment in clinical trials. This involves evaluating the feasibility and efficacy of using both established and novel renal MRI techniques. Additionally, we are active in assessing novel treatments for ADPKD and health care delivery associated with patient benefit at both an individual (personalised) and population level. This includes evaluating quality of life and health economics with the intention of improving the overall patient experience and care.
Current projects focus on:
- Developing translational methods of measuring total kidney volume (TKV) to inform progression
- Identifying a prognostic signature for patients with rapidly progressive ADPKD involving functional MRI techniques
- Evaluating treatment for ADPKD
- Studies on extrarenal manifestations of ADPKD including polycystic liver disease.
Diabetic Kidney Disease (DKD)
DKD is the most common form of CKD and is traditionally classified based on albuminuria and reduced kidney function, but these have limitations as prognostic biomarkers due to the heterogeneity of DKD. Novel prognostic markers are needed to improve stratification of patients based on risk of disease progression. In order to address this question, we are coordinating the iBEAt study, part of the , which aims to determine whether renal imaging biomarkers (MRI and ultrasound) provide insight into the pathogenesis and heterogeneity of DKD, and whether they have potential as prognostic biomarkers in DKD progression. iBEAT is a prospective multi-centre observational cohort study recruiting 500 patients with type 2 diabetes (T2D) and eGFR > 30ml/min/1.73m2. Our role is in coordinating the study but also in setup, quality assurance and processing of the imaging data.
Chronic Liver Disease (CLD)
CLD is a progressive disease characterised by increasing levels of fibrosis, fat accumulation, and ultimately liver cirrhosis potentially leading to liver cancer. We have developed new methods to characterise the effect of CLD on how the liver functions, and are applying those in a number of clinical questions. An important example is in risk assessment for major hepatectomy, a potentially life-saving intervention for patients with liver cancer or liver metastases. Hepatectomy also carries a major risk of severe complications, especially in patients with some form of CLD, and therefore is only offered if this is considered to be safe. We believe our new MRI methods can offer a more accurate assessment of risk and therefore will allow more patients to undergo this treatment in the future. While we are continuing to develop improved methods, we are also running a major clinical study (HEPARIM) aiming to recruit 130 patients selected for major hepatectomy. Our aim is to determine if we can correctly predict post-operative liver function in these patients.
Drug-induced Liver Injury (DILI)
Inhibition of hepatobiliary transporters has been identified as a key initiating mechanism by which drugs may cause DILI. Furthermore, inhibition of hepatic uptake transporters may cause elevated drug concentrations in plasma, while inhibition of biliary efflux transporters can cause intra-hepatocyte drug accumulation; both of these processes could result in DILIs. Noninvasive whole body imaging enables the direct investigation of hepatobiliary function in vivo, and quantification of effects of drugs on hepatobiliary transporter function.
We are a main contributor to the liver work package of the that will validate and make available new liver imaging techniques that will help drug developers bring new, safer, drugs to market and thereby enable doctors to treat their patients more effectively. Our role in TRISTAN is to develop imaging methods for predicting drug toxicity, and evaluate those in preclinical and clinical studies together with our partners in industry and other academic collaborators.
People, Projects & Publications
- People
-
- Susmita Basak (Research fellow, HEPARIM)
- Eve Shalom (PhD student, CFD in liver)
- Bashair al-Hummiany (PhD student, CKD biomarkers)
- Fotis Tagkalakis (PhD student, Renal MRI motion correction)
- Mohamed El-Sharif (MD student, HEPARIM)
- Current Projects / Grants
-
- 10/2020 â 10/2027. NIHR - Efficacy and Mechanism Evaluation Programme. Role: CoI and lead of the central image processing facility.
- 09/2019 â 09/2022. . EPSRC-CASE PhD studentship co-funded by Bayer AG. Role: supervisor.
- 09/2018 â 09/2021. , Medical Research Council, Partnership Grant. Role: CoI and lead of the work package on image processing and quality assurance.
- 01/2018 â 01/2021. (HEPARIM study), Medical Research Council. Role: PI
- 04/2017 â 04/2021. . COST â European Cooperation in Science and Technology. Role: Primary proposer and chair.
- 01/2017 - 01/2022. . Innovative Medicines Initiative. Role: lead of clinical MRI development.
- 09/2016 â 09/2021. . Innovative Medicines Initiative. Role: work package lead.
- Past Projects / Grants
-
- 10/2016 â 10/2019. Functional magnetic resonance imaging to determine severity of cirrhosis in individuals with chronic liver disease. Leeds Anniversary Research Scholarship. Role: Supervisor
- 07/2013 â 12/2017. Optimisation and validation of single-kidney GFR measurement with dynamic contrast-enhanced MRI. Kidney Research UK Project Grant. Role: PI
- 12/2012 â 12/2016. Measuring renal function with dynamic contrast-enhanced MRI: tracer-kinetic model-driven image registration. EPSRC - GlaxoSmithKline, CASE PhD studentship. Role: PI.
- 06/2012 â 06/2014. Measuring liver function and perfusion with DCE-MRI: validation and application to chemoembolization of hepatocellular carcinoma. Great-Britain Sasakawa Foundation Travel Grant. PI
- Recent Publications
-
- Paul Hockings, Nadeem Saeed, Roslyn Simms, Nadia Smith, Matt G Hall, John Waterton, Steven Sourbron. . In: Advances in Magnetic Resonance Technology and Applications, Eds. Seiberlich & Gulani, Elsevier 2020 1:Iiii-Ixxxvi.
- Roslyn Simms and Steven Sourbron. . Nephrol Dial Transplant. 2020 Jun 1;35(6):915-919.
- Kazuhiro Saito, Joseph Ledsam, Steven Sourbron, Yoichi Araki. . Quantitative Imaging in Medicine and Surgery. 2020 Jun;10(6):1298-1306.
- Kim M Gooding, Chrysta Lienczewski, Massimo Papale, Niina Koivuviita, Marlena Maziarz, Anna-Maria Dutius Andersson, Kanishka Sharma, Paola Pontrelli, Alberto Garcia Hernandez, Julie Bailey, Kay Tobin, Virva Saunavaara, Anna Zetterqvist, David Shelley, Irvin Teh, Claire Ball, Sapna Puppala, Mark Ibberson, Anil Karihaloo, Kaj MetsÃĪrinne, Rosamonde Banks, Peter S Gilmour, Michael Mansfield, Mark Gilchrist, Dick de Zeeuw, Hiddo JL Heerspink, Pirjo Nuutila, Matthias Kretzler, Matthew Wellberry-Smith, Loreto Gesualdo, Dennis Andress, Nicolas Grenier, Angela C Shore, Maria F Gomez, Steven Sourbron. . BMC Nephrol. 2020 Jun 29;21(1):242.
- Andreas Pohlmann, Susan J. Back, Andrea Fekete, Iris Friedli, Stefanie Hectors, Neil Peter Jerome, Min-Chi Ku, Dario Livio Longo, Martin Meier, Jason M. Millward, JoÃĢo S. Periquito, Erdmann Seeliger, Suraj D. Serai, Sonia Waiczies, Steven Sourbron, Christoffer Laustsen, Thoralf Niendorf. ; Methods Mol Biol. 2021;2216:3-23.
- Hockings, P., Laustsen, C., Joles, J., Mark, P. and Sourbron, S. . Magn Reson Mater Phy. 2020 Feb;33(1):1-2.
- Ilona Alexandra Dekkers, MD MSc; Anneloes de Boer, MD; Kaniska Sharma, PhD; Eleanor Cox, PhD; Hildo Lamb, MD PhD; David L Buckley, PhD; Octavia Bane, PhD; David M Morris, PhD; Pottumarthi V Prasad, PhD; Scott IK Semple, PhD; Keith A Gillis, MBChB PhD; P Hockings, PhD; Charlotte Buchanan, PhD; Markus Wolf, MD; Christoffer Laustsen, PhD; Tim Leiner, MD PhD; B Haddock, PhD; Hans Hogenduin, PhD; Pim Pullens, PhD; Steven Sourbron, PhD; Susan Francis, PhD. . Magn Reson Mater Phy. 2020 Feb;33(1):163-176.
- Octavia Bane, PhD; Iosif A. Mendichovszky, MD, PhD; Bastien Milani, PhD; Ilona A. Dekkers, MD, MS; Jean-Francois Deux, MD, PhD; Per Eckerbom, MD; Nicolas Grenier, MD; Michael E. Hall, MD, MS; Tsutomu Inoue, MD, PhD; Christoffer Laustsen, PhD; Lilach O. Lerman, MD, PhD; Chunlei Liu, PhD; Glen Morrell, MD, PhD; Michael Pedersen, PhD; Menno Pruijm, MD; Elizabeth A. Sadowski, MD; Erdmann Seeliger, PhD; Kanishka Sharma, PhD; Harriet C Thoeny, MD; Peter Vermathen, PhD; Zhen J. Wang, MD; Zbigniew Serafin, MD, PhD; Jeff L. Zhang, PhD; Susan T. Francis, PhD; Steven Sourbron, PhD; Andreas Pohlmann, PhD; Sean B. Fain, PhD; Pottumarthi V. Prasad, PhD. . Magn Reson Mater Phy. 2020 Feb;33(1):199-215.
- Fabio Nery, PhD; Charlotte E. Buchanan, PhD; Anita A. Harteveld, PhD; Aghogho Odudu, PhD; Octavia Bane, PhD; Eleanor F. Cox, PhD; Katja Derlin, MD; H. Michael Gach, PhD; Xavier Golay, PhD; Marcel Gutberlet, PhD; Christoffer Laustsen, PhD; Alexandra Ljimani, MD; Ananth J. Madhuranthakam, PhD; Ivan Pedrosa, MD, PhD; Pottumarthi V. Prasad, PhD; Philip M. Robson, PhD; Kanishka Sharma, PhD; Steven Sourbron, PhD; Manuel Taso, PhD; David L. Thomas, PhD; Danny J.J. Wang, PhD; Jeff L. Zhang, PhD; David C. Alsop, PhD; Sean B. Fain, PhD; Susan T. Francis, PhD; MarÃa A. FernÃĄndezâSeara, PhD. . Magn Reson Mater Phy. 2020 Feb;33(1):141-161.
- Alexandra Ljimani, MD, BSc; Anna Caroli, PhD; Christoffer Laustsen, PhD; Susan Francis, PhD; Iosif Alexandru Mendichovszky, MD, PhD; Octavia Bane, PhD; Fabio Nery, PhD; Kanishka Sharma, PhD; Andreas Pohlmann, PhD; Ilona A Dekkers, MD, Msc; Vallee Jean-Paul, MD, PhD; Katja Derlin, MD; Mike Notohamiprodjo, MD; Ruth P Lim, MBBS, MMed, MS; Stefano Palmucci, MD; Suraj D Serai, PhD; Joao Periquito, MsC; Zhen Jane Wang, MD; Martijn Froeling, PhD; Harriet C Thoeny, MD; Pottumarthi V Prasad, PhD; Moritz Schneider, PhD; Thoralf Niendorf, PhD; Pim Pullens, PhD; Steven Sourbron, PhD; Eric E Sigmund, PhD. . Magn Reson Mater Phy. 2020 Feb;33(1):177-195.
- Iosif Mendichovszky, Pim Pullens, Ilona Dekkers, Fabio Nery, Octavia Bane, Andreas Pohlmann, Anneloes de Boer, Alexandra Ljimani, MD, Aghogho Odudu, Charlotte Buchanan, Kanishka Sharma, Christoffer Laustsen, Anita Harteveld, Xavier Golay, Ivan Pedrosa, David Alsop, Sean Fain, Anna Caroli, Pottumarthi Prasad, Susan Francis, Eric Sigmund, Maria Seara-Fernandez, Steven Sourbron. . Magn Reson Mater Phy. 2020 Feb;33(1):131-140.
- Benjamin Taton; Renaud De La Faille, MD; Julien Asselineau, MSc; Paul Perez, MD, PhD; Pierre Merville, MD, PhD; Thierry Colin, PhD; Christian Combe, MD, PhD; Steven Sourbron, PhD; Nicolas Grenier, MD, PhD. . Eur J Radiol. 2019 Aug;117:209-215.
- Susmita Basak, David L Buckley, Constantina Chrysochou, Anita Banerji, Diana Vasallo, Aghogho Odudu, Philip A Kalra, S Sourbron. . Magn Reson Imaging. 2019 Jun;59:53-60.
- J. Gerry Kenna, John C. Waterton, Andreas Baudy, Aleksandra Galetin, Catherine D. G. Hines, Paul Hockings, Manishkumar Patel, Daniel Scotcher, S. Sourbron, Sabina Ziemian, Gunnar Schuetz. , Eds: Minjun Chen, Yvonne Will, Humana Press, New York 2018 1:627-651.
- Nicholas M Selby, Peter J Blankestijn, Peter Boor, Christian Combe, Kai-Uwe Eckardt, Eli Eikefjord, Nuria Garcia-Fernandez, Xavier Golay, Isky Gordon, Nicolas Grenier, Paul D Hockings, Jens Jenson, Jaap A Joles, Philip A Kalra, Bernhard K KrÃĪmer, Patrick B Mark, Iosif A Mendichovszky, Olivera Nikolic, Aghogho Odudu, Albert CM Ong, Alberto Ortiz, Menno Pruijm, Giuseppe Remuzzi, Jarle RÃļrvik, Sophie de Seigneux, Roslyn J Simms, Janka Slatinska, Paul Summers, Maarten W Taal, Harriet C Thoeny, Jean-Paul VallÃĐe, Marcos Wolf, Anna Caroli, S. Sourbron. . Nephrol Dial Transplant 2018 Sep 1;33(suppl_2):ii4-ii14.
- Kazuhiro Saito, Joseph Ledsam, Katsutoshi Sugimoto, S. Sourbron, Yoichi Araki, Koichi Tokuuye. . J Belg Soc Radiol. 2018 Apr 20;102(1):40.
- Tze Min Wah, S. Sourbron, Daniel Jonathan Wilson, Derek Magee, Walter Martin Gregory, Peter John Selby, David L. Buckley. . Diagnostics (Basel). 2018 Jan 8;8(1):3.




