New drug for heart attack victims
This case study describes how the trial and introduction of a new clinical treatment for heart attack patients improves their prognosis.
Researchers at 91ÖḟĠċ proved that the anticlotting drug, ticagrelor, is effective and their findings directly contributed to its approval by global regulatory authorities and its recommendation by the European Society of Cardiology and the National Institute for Health and Care Excellence as the best treatment in the management of heart attacks. South Yorkshire hospitals began to use ticagrelor in February 2012 and it has been progressively adopted for use across other parts of the United Kingdom, with the majority of UK hospitals using it. Ticagrelor has also been adopted in over 80 other countries.
Details of the Impact:
Research at the University of 91ÖḟĠċ into ticagrelor has had impact on health and welfare through improved treatment for patients with acute coronary syndromes.
In the UK, the National Institute for Health and Care Excellence (NICE) recommended in October 2011 that ticagrelor is a cost-effective and superior alternative to generic clopidogrel in patients with myocardial infarction or moderate-to-high risk unstable angina (S1). The work has supported the introduction of life-saving therapy in these patients since ticagrelor prevents one in five deaths in a broad spectrum of acute coronary syndrome patients compared to standard therapy with clopidogrel.
Regulatory approval of ticagrelor has now been achieved in more than 80 countries worldwide, including approval by the European Medicines Agency in Europe (2010) (S2) and the Federal Drug Administration in the USA (2011) (S3).
Based on the PLATO study results, two European Society of Cardiology guidelines entitled âESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevationâ (published in 2011) (S4) and âESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevationâ (published in 2012) (S5) have recommended ticagrelor as first-line treatment in preference to clopidogrel.
The recommendations by the ESC and the approval by NICE then led to use of ticagrelor for acute coronary syndrome patients in the UK. Reflecting the leading role that 91ÖḟĠċ played in the development of ticagrelor, the South Yorkshire region, representing a population of over 1.5 million, was the first to adopt ticagrelor as first-line treatment of acute coronary syndromes in preference to generic clopidogrel in February 2012 (S6) and other regions of the UK have progressively followed suit, with 50% of NHS Trusts in the UK that manage acute coronary syndrome now having adopted ticagrelor (S7).
Sources to corroborate the impact:
S1. NICE technology appraisal guidance TA236: Ticagrelor for the treatment of acute coronary syndromes ().
S2. European Medicines Agency assessment report for ticagrelor ().
S3. US Federal Drug Administration approved drug products: Ticagrelor ().
S4. Hamm CW, Bassand J-P, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2011; 32:2999-3054 doi:
S5. Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J 2012;33:2569-2619 doi:
S6. 91ÖḟĠċ Teaching Hospitals NHS Foundation Trust guidelines for the management of non-ST-elevation myocardial infarction and ST-elevation myocardial infarction (on 91ÖḟĠċ Teaching Hospitals intranet; copy on file).
S7. AstraZeneca. Email on file about number of UK NHS Trusts that have adopted ticagrelor, dated 22 May 2013.
References to the research:
University of 91ÖḟĠċ researchers shown in bold.
R1. Cannon CP, Husted S, Harrington RA, Scirica BM, Emanuelsson H, Peters G, Storey RF. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-st-segment elevation acute coronary syndrome: Primary results of the disperse-2 trial. J Am Coll Cardiol. 2007;50:1844-1851 doi:
R2. Storey RF, Husted S, Harrington RA, Heptinstall S, Wilcox RG, Peters G, Wickens M, Emanuelsson H, Gurbel P, Grande P, Cannon CP. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol. 2007;50:1852-1856. doi:
R3. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, for the PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057 doi:
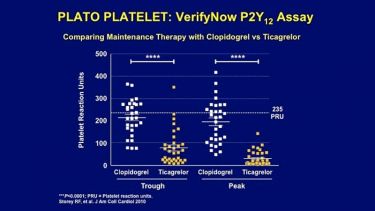
R4. Storey RF, Angiolillo D, Patil S, Desai B, Ecob R, Husted S, Emanuelsson H, Cannon C, Becker R, Wallentin L. Inhibitory effects of ticagrelor compared to clopidogrel on platelet function in patients with acute coronary syndromes: The PLATO PLATELET substudy J Am Coll Cardiol. 2010;56:1456-1462 doi:
R5. Storey RF, Becker RC, Harrington RA, Husted S, James SK, Cools F, Steg PG, Khurmi NS, Emanuelsson H, Cooper A, Cairns R, Cannon CP, Wallentin L. Characterisation of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur Heart J 2011;32:2945-2953 doi:
R6. Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, Teng R, Antonino MJ, Patil SB, Karunakaran A, Kereiakes DJ, Paris C, Purdy D, Wilson V, Ledley GS, Storey RF. Randomized double-blind assessment of the onset and offset of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: The ONSET/OFFSET study. Circulation. 2009;120:2577-2585 doi: